Colonoscopy, Cologuard, and Liquid Biopsy: The Evolving Landscape of Colorectal Cancer Screening
From enduring the infamous prep to exploring non-invasive alternatives, here's what you need to know about detecting and preventing colorectal cancer
Preparing for a colonoscopy is like gearing up for the worst endurance challenge ever—except instead of carb-loading, you're downing laxatives like a pro. Sure, it’s a vital test for catching colorectal cancer early, but that doesn’t make the prep any less of a wild ride. Between surviving on clear liquids, experiencing “jet propulsion” like never before, and realizing your bathroom is now your official home office, you can’t help but think—this better be worth it!
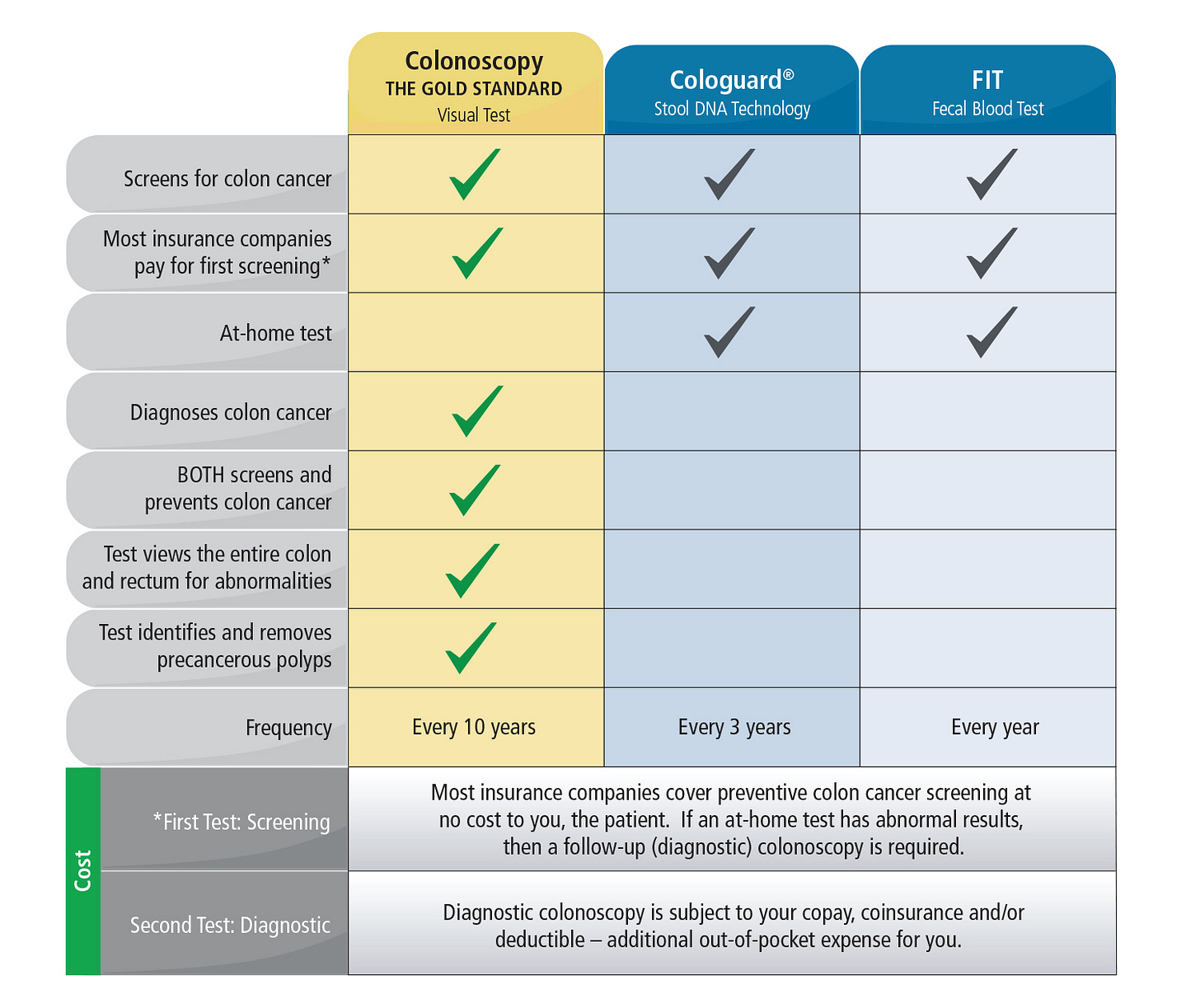
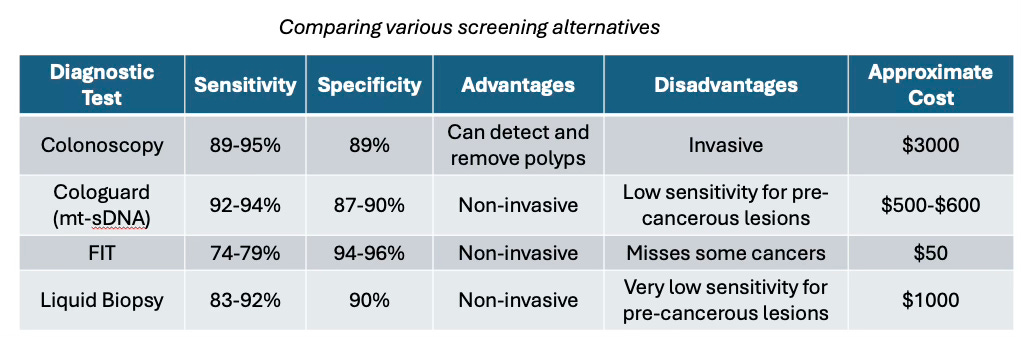
Fortunately (or unfortunately), colonoscopy is the gold standard for colorectal cancer (CRC) screening. By allowing direct visualization of the entire colon with a flexible tube and camera, it not only detects precancerous polyps but also removes them in the same procedure—something no other diagnostic test can offer. In fact, immediate polypectomy1 has been shown to reduce CRC incidence by up to 53%. While colonoscopy boasts high sensitivity (95%) and specificity (92%) for detecting high-risk lesions, it does come with drawbacks. It is invasive, requires thorough bowel preparation, and carries a small risk of complications2. However, for patients with negative results, it provides a long 10–15-year screening interval. The primary reason is that the adenoma-carcinoma sequence, which is the stepwise progression of genetic mutations leading to CRC, is estimated to take at least 10 years. Additionally, its success is highly operator-dependent, with adenoma detection rates varying by endoscopist skill—leading to a 30–40% miss rate for small polyps.
Colorectal cancer (CRC) is the second-most common cause of cancer-related death in the United States. However, despite the established benefits of CRC screening, screening rates in the United States remain below the National Colorectal Cancer Roundtable goal of 80%.
Recent trends reveal a disturbing rise in CRC cases among young adults, teenagers, and even children. Since the 1990s, CRC incidence in individuals aged mid-20s to late 50s has more than doubled. Alarmingly, in 2019, 20% of all CRC diagnoses were in patients under 55. Even more concerning is the staggering 185% increase among young adults aged 20–24. While the exact cause remains unclear, contributing factors likely include lifestyle choices such as poor diet, obesity, smoking, and heavy alcohol consumption, as well as shifts in the gut microbiome and environmental influences. In response to these concerning trends, the U.S. Preventive Services Task Force (USPSTF) updated its guidelines in May 2021, lowering the recommended age for colorectal cancer (CRC) screening from 50 to 45 for average-risk adults. CRC is a serious threat to the young population, and it is the most commonly diagnosed and the most common cause of cancer death among men younger than 50 in the United States. A lack of screening in this age group implies that screening might significantly improve the burden of disease. According to this study, adherence to colorectal cancer (CRC) screening—including colonoscopy and other methods—is approximately 40% among individuals aged 50 to 54. Unfortunately, the adherence rate for colonoscopy is lower than desired due to fear and anxiety, preparation challenges, time commitment3, and lack of awareness.
Fortunately, non-invasive diagnostic tests for CRC have emerged as promising options to improve screening compliance, and is a reasonable option for those reluctant to undergo colonoscopy. The fecal immunochemical test (FIT) that detects hidden (occult) blood in the stool, has largely replaced older stool-based tests due to its high sensitivity, specificity, and low cost. Multi-target stool DNA (mt-sDNA) tests like Cologuard, that detects both DNA markers4 and hemoglobin in stool samples, have shown superior sensitivity (94% detection rate compared to FIT’s ~70%) in detecting CRC and advanced precancerous lesions compared to FIT (43% vs. 23%), though at a higher cost. For instance, Cologuard has been used more than 17 million times since its launch in 2014. This at-home FDA-approved stool test has significantly increased screening compliance, contributing to 77% of the rise in colorectal cancer screening rates from 2018 to 2021. In fact, data from the Centers for Disease Control and Prevention show that Cologuard use is the primary factor behind the increase in colon cancer screening rates from 63% in 2015 to 72% in 2021 among Americans ages 50-75. The test’s observed adherence rate of 71% nearly doubles that of colonoscopy (38%), demonstrating its effectiveness in improving screening participation among previously non-adherent patients.
However, it is important to note that Cologuard is only approved for screening average-risk adults aged 50 to 75 and must be repeated every three years if you choose it over a colonoscopy. If you have a family history of colon or rectal cancer, polyps, Lynch syndrome, or conditions such as inflammatory bowel disease such as ulcerative colitis or Crohn's, iron deficiency anemia, or bleeding hemorrhoids, you are considered high-risk and should undergo a colonoscopy instead. Furthermore, if your Cologuard test comes back with a positive result, a colonoscopy will still be necessary to confirm a positive diagnosis.
Blood-based liquid-biopsy tests which look for ctDNA (circulating tumor DNA) in the blood, such as the recently approved Shield test, offer yet another non-invasive screening option. It requires only a simple blood draw, which is less invasive and more convenient than collecting a stool sample. However, its sensitivity for CRC (83%) and advanced precancerous lesions (13%) is lower than Cologuard, and therefore not the best non-invasive first-line screening option. However, its minimally invasive approach can potentially enable frequent monitoring for disease recurrence or treatment resistance .
Liquid biopsy offers a powerful advantage in detecting minimal residual disease (MRD) and predicting cancer recurrence earlier than traditional methods. For example, the Guardant Reveal blood test has demonstrated high sensitivity and specificity in identifying colorectal cancer (CRC) recurrence at multiple post-surgical time points. Beyond early detection, blood-based liquid biopsy may also play a crucial role in guiding targeted treatments. By capturing tumor heterogeneity and tracking changes in mutation status over time, it provides valuable insights for selecting the most effective therapies and monitoring potential resistance.
Takeaway: Colonoscopy remains the gold standard for colorectal cancer (CRC) screening, but its invasive nature and prep challenges deter many. Fortunately, non-invasive options like FIT, Cologuard, and liquid biopsy tests offer promising alternatives to improve screening adherence. With CRC rates rising among younger adults, increasing awareness and accessibility of screening methods is crucial for early detection and prevention.
Polypectomy is a surgical procedure to remove polyps, which are abnormal growths of tissue.
There are risks of perforation (0.1% of cases) and bleeding.
The procedure often requires taking time off work and arranging transportation.
Detects 5-11 distinct CRC-associated biomarkers (KRAS mutations), including altered DNA.